The RESORCE trial evaluated the efficacy and safety of STIVARGA® (regorafenib)1,2
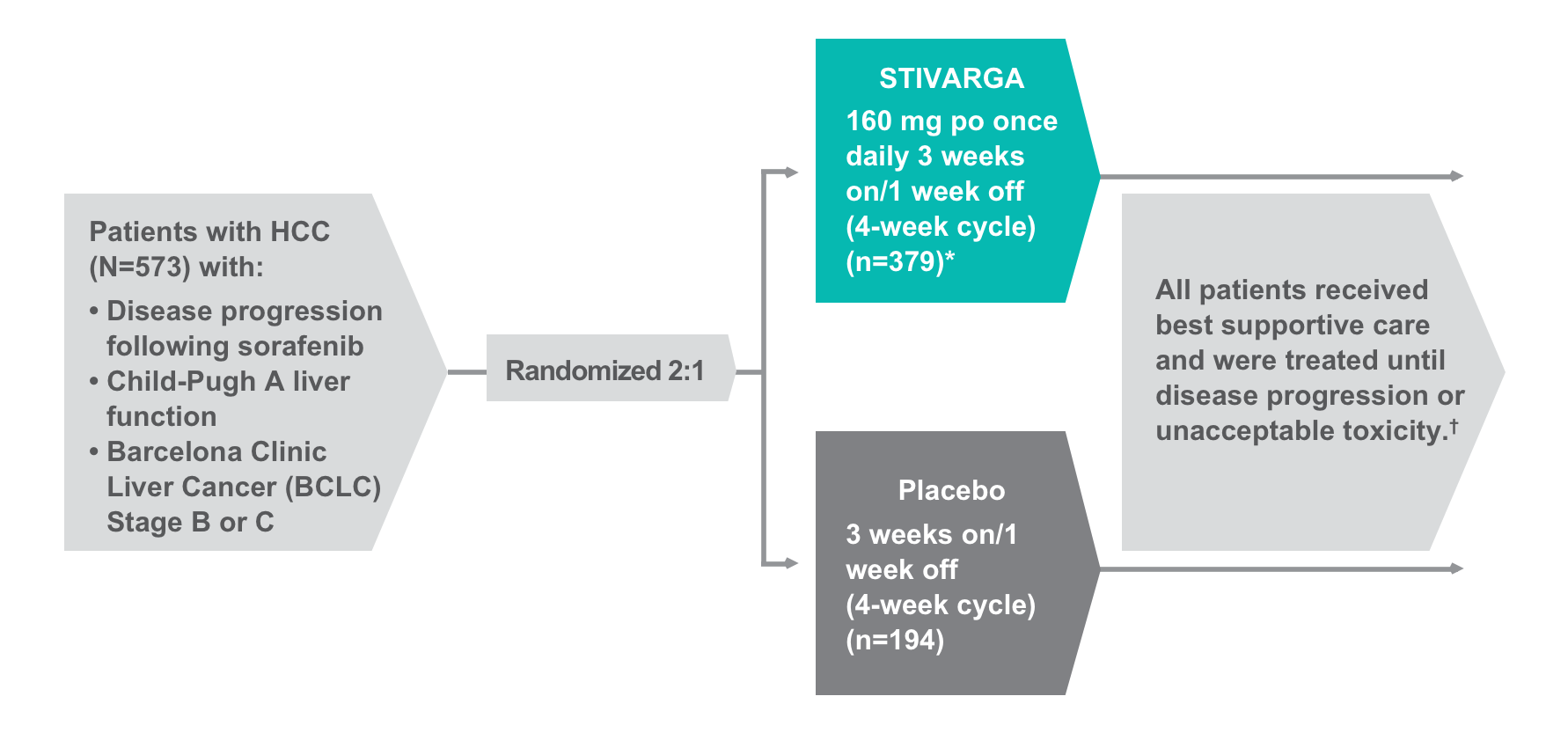
REgorafenib after SORafenib in patients with hepatoCEllular carcinoma (RESORCE) was an international, multicenter, randomized (2:1), double-blind, placebo-controlled phase 3 trial that evaluated the efficacy and safety of STIVARGA in HCC patients with progression following sorafenib (N=573).
- Patients who permanently discontinued sorafenib due to toxicity or who were unable to tolerate sorafenib doses of 400 mg once daily were ineligible for the trial
Primary endpoint1,2:
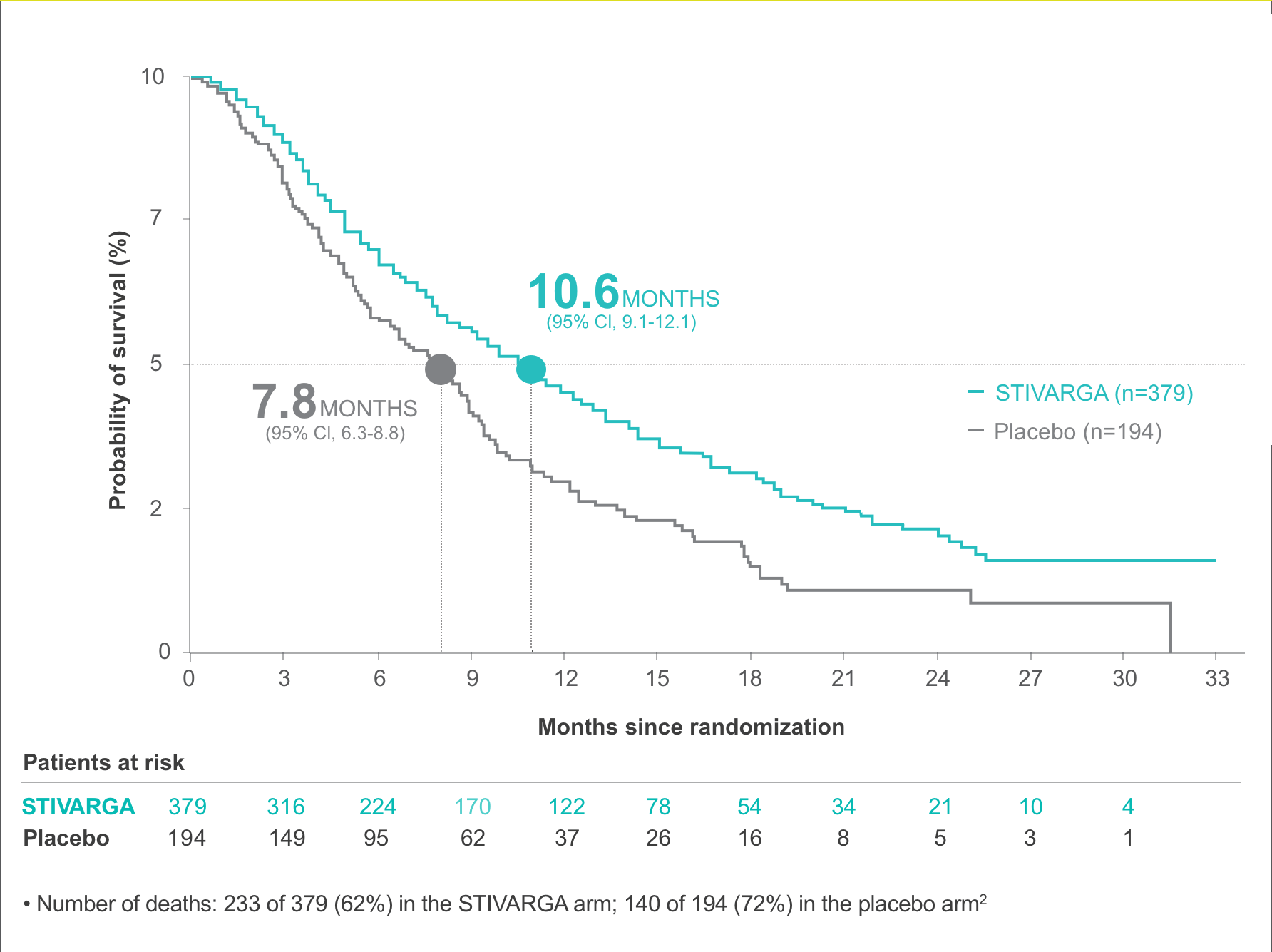
- Overall survival (OS), analyzed by intention to treat
Secondary endpoints1,2:
- Progression-free survival (PFS), time to progression, overall response rate (ORR; patients with complete response [CR] or partial response [PR]), disease control rate (DCR; patients with CR, PR, or stable disease [SD] for ≥6 weeks)
- Analyzed by intention to treat; assessed using modified Response Evaluation Criteria In Solid Tumors (mRECIST) and RECIST 1.1
Tertiary endpoint1,2:
- Duration of response
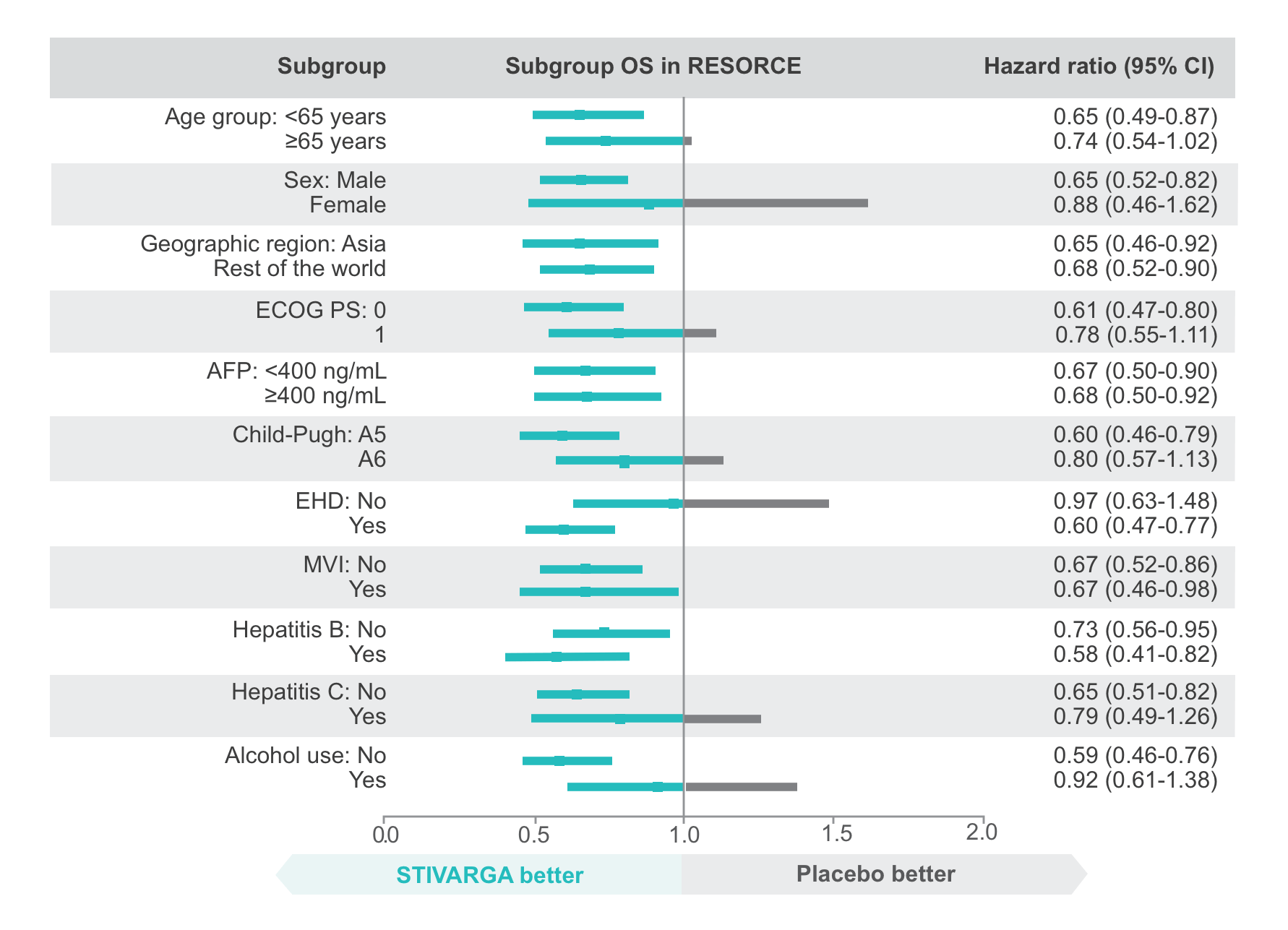
Patients in the RESORCE trial had good performance status and preserved liver function1,2
Baseline patient characteristics were similar between each arm1,2
| STIVARGA (n=379) % | Placebo (n=194) % | |
|---|---|---|
| Sex | ||
| Male | 88 | 88 |
| Female | 12 | 12 |
| Race | ||
| White | 36 | 35 |
| Asian‡ | 41 | 40 |
| Black | 2 | 1 |
| Other/Not reported | 21 | 24 |
| Median age, years | 64 | 62 |
| Eastern Cooperative Oncology Group performance status (ECOG PS) | ||
| 0 | 65 | 67 |
| 1 | 35 | 33 |
| Macrovascular invasion (MVI) | 29 | 28 |
| Extrahepatic disease (EHD) | 70 | 76 |
| MVI and/or EHD | 80 | 84 |
| Alpha-fetoprotein (AFP) ≥400 ng/mL | 43 | 45 |
| Child-Pugh§ | ||
| A | 98 | 97 |
| B | 1 | 3 |
| BCLC stage | ||
| A | <1 | 0 |
| B | 14 | 11 |
| C | 86 | 89 |
| Liver cirrhosis (investigator assessed) | 75 | 74 |
| Etiology of HCCII | ||
| Hepatitis B | 38 | 38 |
| Alcohol use | 24 | 28 |
| Hepatitis C | 21 | 21 |
| Unknown | 17 | 16 |
| Nonalcoholic steatohepatitis | 7 | 7 |
| Other | 7 | 5 |
| Duration of sorafenib treatment, months | 7.8 (4.2-14.5) | 7.8 (4.4-14.7) |
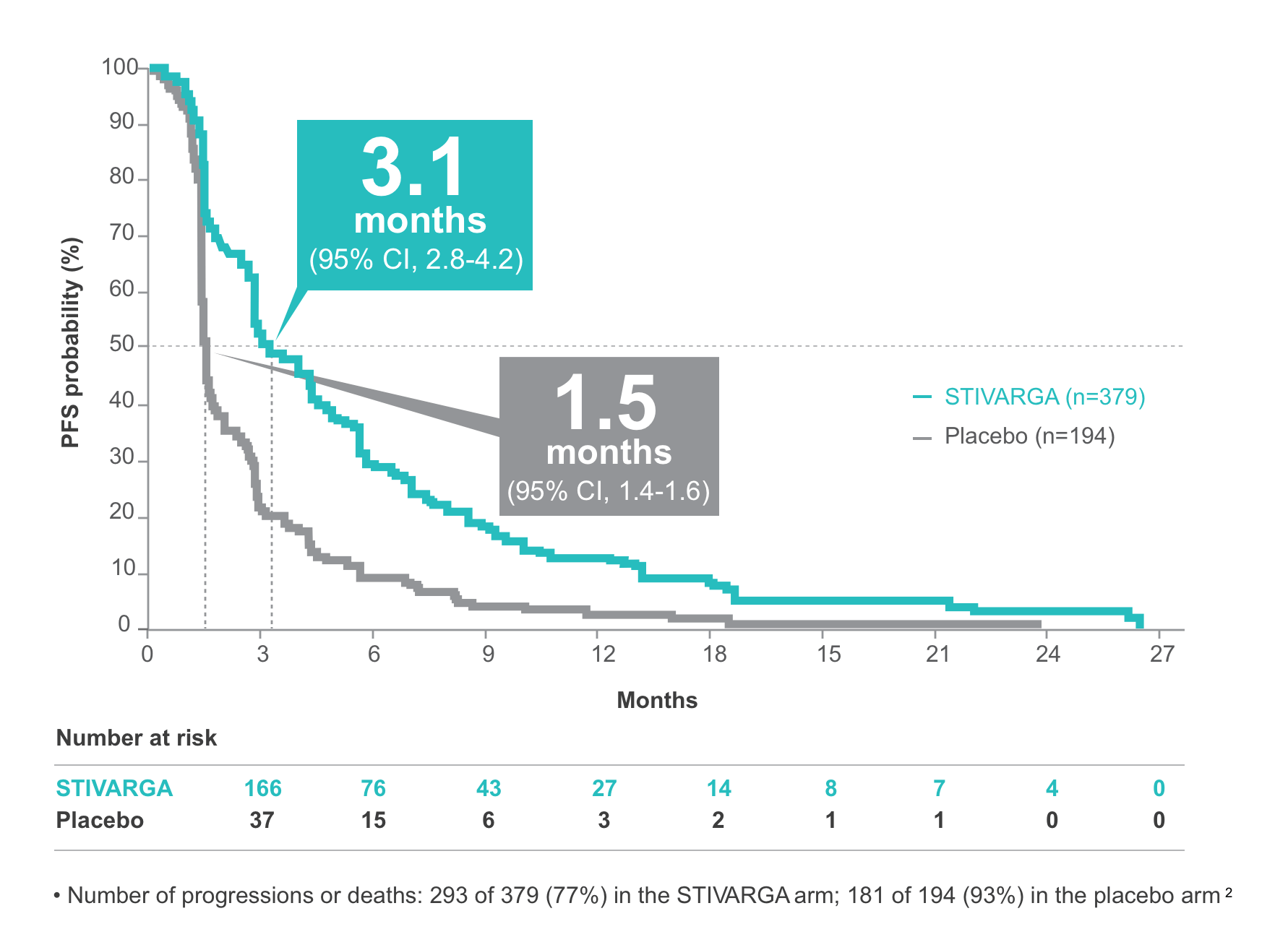
STIVARGA more than doubled PFS vs placebo1,2
3.1-month median PFS achieved with STIVARGA vs 1.5 months with placebo
Reduction in risk of progression or death with STIVARGA was consistent when assessed by RECIST 1.1
- 3.4-month (95% CI, 2.9-4.2) median PFS achieved with STIVARGA vs 1.5 months (95% CI, 1.4-1.5 with placebo (HR: 0.43; 95% CI, 0.35-0.52) (RECIST 1.1)
- Number of progressions or deaths: 288 of 379 (76%) in the STIVARGA arm; 184 of 194 (95%) in the placebo arm
DCR, CR + PR + SD; ORR, CR + PR.