Standard starting dose with options for dose management
STIVARGA® (regorafenib) dosing in the CORRECT, phase 3 trial
The recommended starting dose is 160 mg STIVARGA (four 40-mg tablets) taken orally once daily for the first 3 weeks, followed by a 1-week treatment break.1
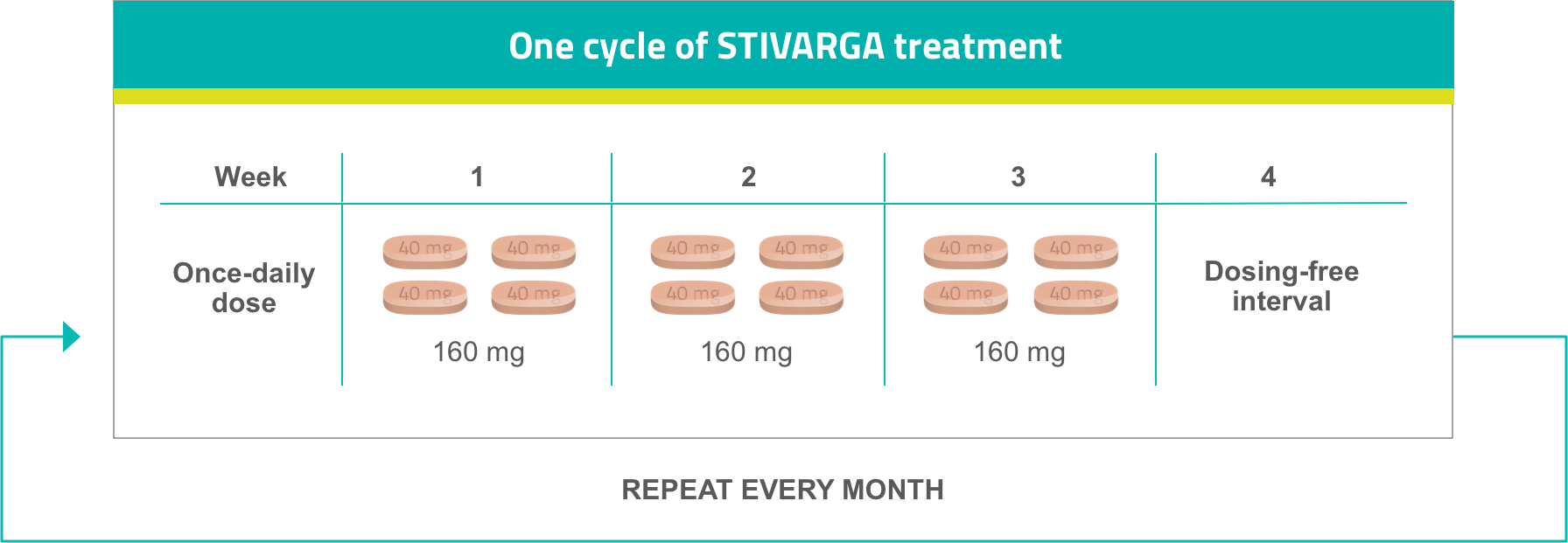
Dosage and administration for STIVARGA1
- Treatment should continue until disease progression or until unacceptable toxicity occurs
- STIVARGA should be taken whole with water after a low-fat meal that contains <600 calories and <30% fat at the same time each day
- Advise patients to take any missed dose on the same day, as soon as they remember, and that they must not take 2 doses on the same day to make up for a dose missed on the previous day
- No dose adjustment is recommended for patients with renal impairment
- The pharmacokinetics of STIVARGA have not been studied in patients who are on dialysis and there is no recommended dose for this patient population
- No dose adjustments are required based on mild (total bilirubin ≤ upper limit of normal (ULN) and aspartate aminotransferase (AST) >ULN, or total bilirubin >ULN to ≤1.5x ULN) or moderate (total bilirubin >1.5 to ≤3x ULN and any AST) hepatic impairment. Closely monitor patients with hepatic impairment for adverse reactions. STIVARGA is not recommended for use in patients with severe hepatic impairment (total bilirubin >3x ULN), as STIVARGA has not been studied in this population
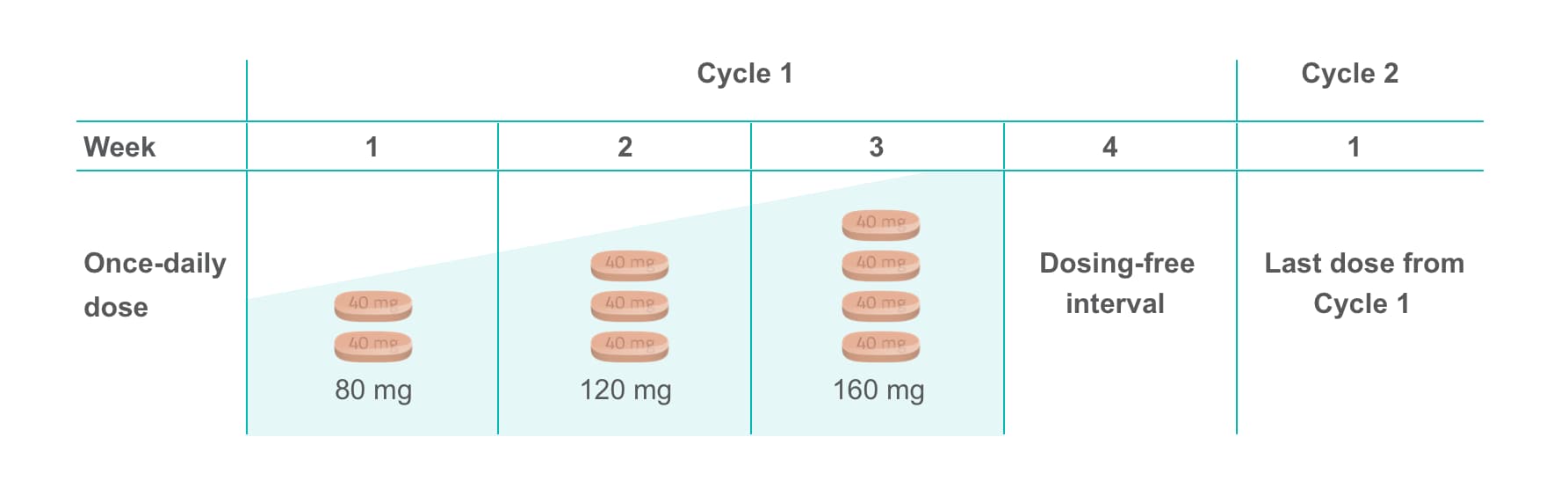
The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) include the option for the following dose-escalation schedule (Category 2A)2,3*†
Regorafenib (STIVARGA®) dose escalation schedule in mCRC
National Comprehensive Cancer Network (NCCN®) makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
*The study supporting the dose-escalation schedule has not been reviewed by the FDA. The study was a randomized, phase 2, US-based trial through the Academic and Community Cancer Research United (ACCRU) research network that looked at the proportion of patients who completed 2 cycles of STIVARGA and initiated a third cycle (N=116). The efficacy of the alternative dosing schedule cannot be compared to the efficacy of other trials. 4
†Category 2A: Based upon lower-level evidence, there is uniform NCCN consensus that the intervention is appropriate.2, 3
AE, adverse event; FDA, US Food and Drug Administration.
- Dosing escalation takes place during the first 4-week cycle (3 weeks on, 1 week off)2,3
- Week 1: 80 mg of STIVARGA (two 40-mg tablets) taken orally once daily, Week 2: 120 mg (three 40-mg tablets), Week 3 dose is 160 mg (four 40-mg tablets), followed by Week 4 dose-free interval1-3
- Subsequent cycles are dosed at the last dose from Cycle 12,3


