Harness the proven efficacy of STIVARGA to significantly improve progression-free survival in previously treated patients with GIST1,2
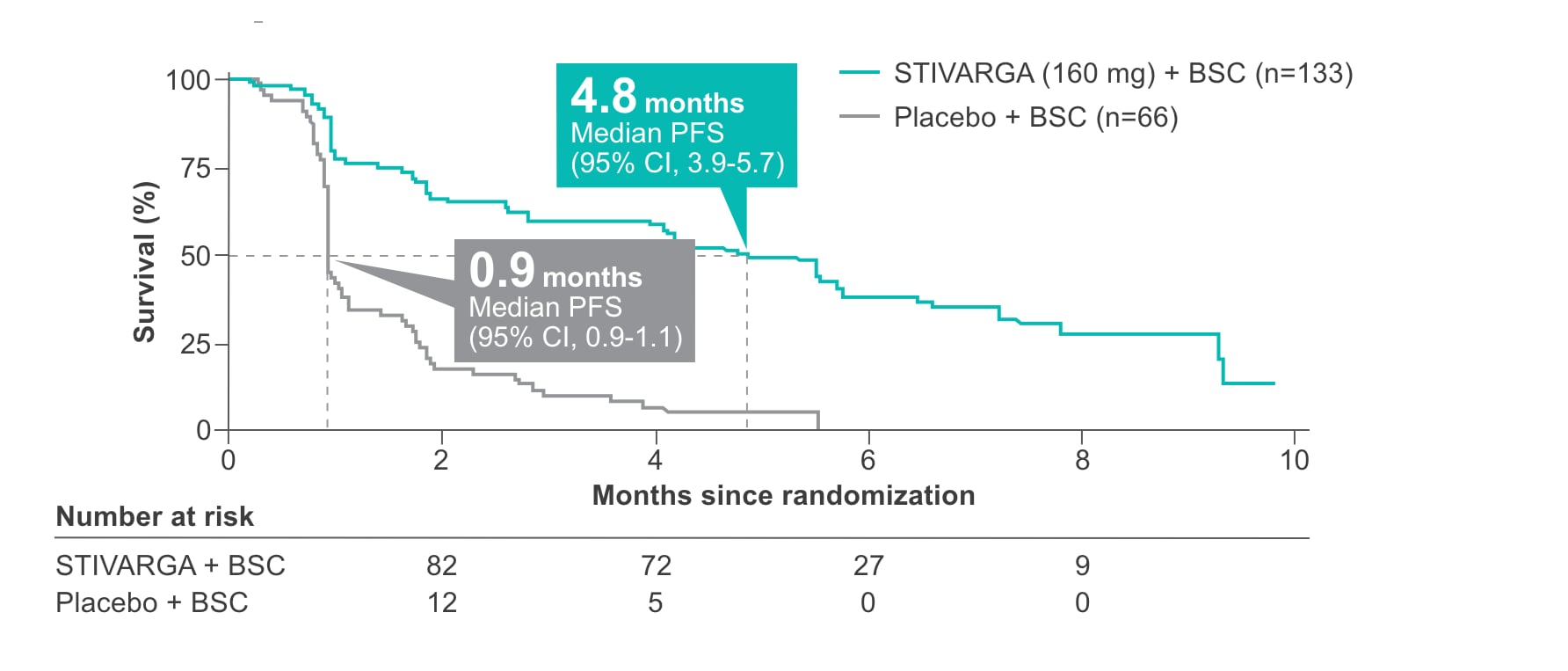
In the pivotal GRID trial, STIVARGA demonstrated statistically significant improvement in PFS in previously treated patients with GIST1,2
- 82 of 133 STIVARGA patients (62%) vs 63 of 66 placebo patients (96%) experienced disease progression or died2
- At the time of disease progression as assessed by central review, the study blind was broken and all patients were offered the opportunity to take STIVARGA at the investigator’s discretion. Cross-over to open-label STIVARGA occurred in 58 (88%) placebo-treated patients after disease progression2
- There was no statistically significant difference in OS at the final OS analysis, conducted at 162 OS events2
- DCR was 52.6% with STIVARGA (70/133 patients) vs 9.1% with placebo (6/66 patients)1
- DCR was a secondary endpoint
- DCR is defined as the rate of complete response or partial response plus stable disease lasting for ≥12 weeks1
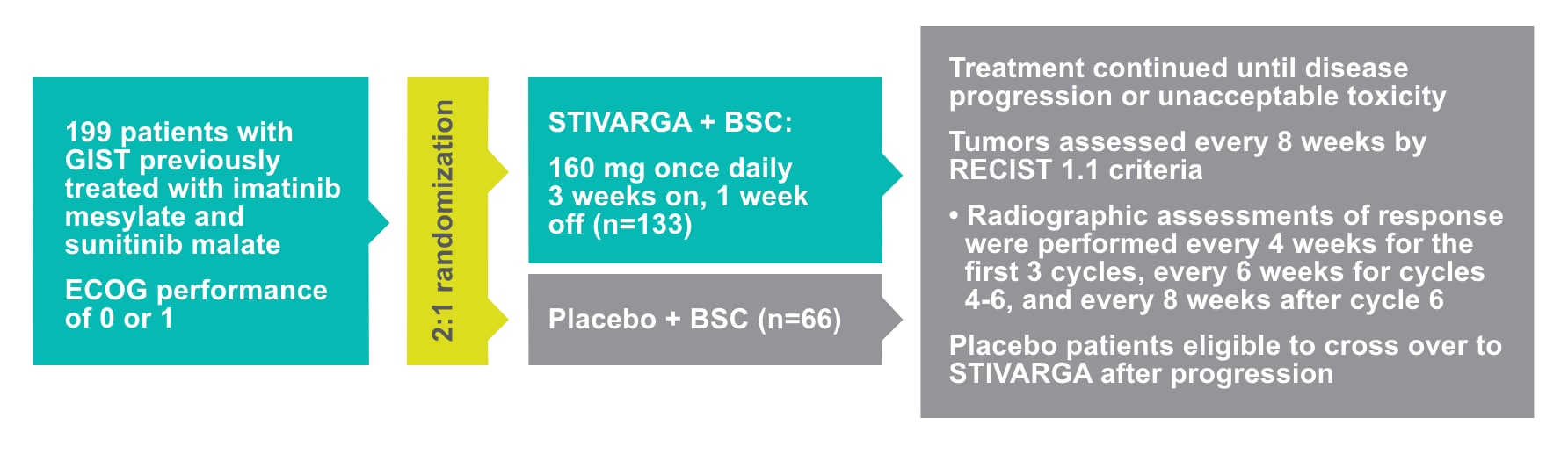
GRID trial study design
STIVARGA was studied in patients with a high unmet need following disease progression on 2 prior TKIs in GRID1
- The GRID trial was a phase III, randomized, placebo-controlled trial in patients with metastatic or unresectable GIST who had progressed after failure of at least imatinib and sunitinib or after intolerance to imatinib1,2
- Primary endpoint: (PFS)2
- Based on disease assessment by independent radiological review using modified RECIST 1.1. criteria, in which lymph nodes and bone lesions were not target lesions and progressively growing new tumor nodule within a pre-existing tumor mass was progression
- Key secondary endpoint: overall survival (OS). Secondary endpoints included OS, time to progression, objective response rate, and disease control rate (DCR; defined as rate of complete response or partial response plus stable disease lasting for ≥12 weeks), safety, and tolerability1,2
- At progression, patients on placebo could cross over to receive open-label STIVARGA, and patients originally assigned to the STIVARGA group could continue to receive open-label therapy2
GRID trial patient population
Patient population with a high unmet need following the failure of 2 prior TKIs. Demographics were similar between arms.1
GRID patient population1
| STIVARGA (n=133) | Placebo (n=66) | |
|---|---|---|
| Median age, y | 60 | 61 |
| Sex | ||
| Male | 64% | 64% |
| Female | 36% | 36% |
| Ethnic group | ||
| White | 68% | 68% |
| Black or African American | 0% | 2% |
| Asian | 26% | 24% |
| Not reported or missing | 7% | 6% |
| Performance status | ||
| ECOG 0 | 55% | 56% |
| ECOG 1 | 45% | 44% |