Guidance on HFSR management
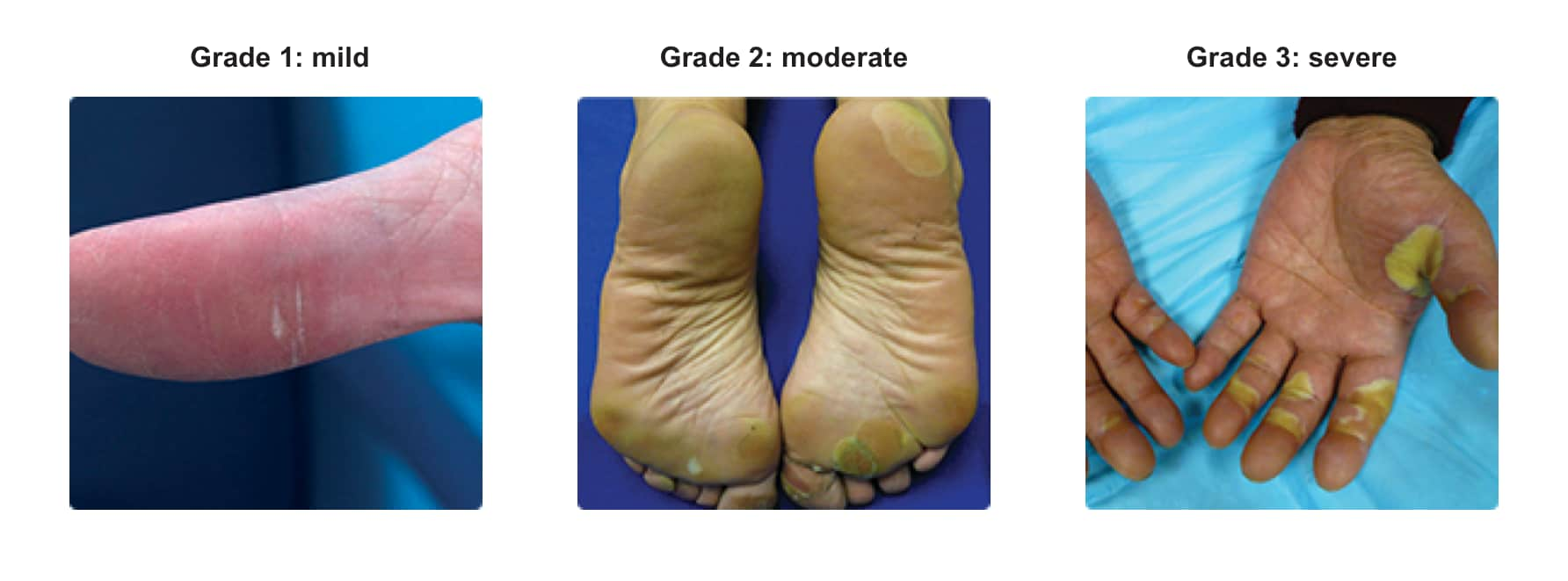
Dermatological toxicity1
- In randomized, placebo-controlled trials, adverse skin reactions occurred in 71.9% of patients in the STIVARGA® (regorafenib) arm and in 25.5% of patients in the placebo arm, including hand-foot skin reaction (HFSR) also known as palmar-plantar erythrodysesthesia syndrome, and severe rash requiring dose modification
- In the randomized, placebo-controlled trials, the overall incidence of HFSR was higher in 1142 STIVARGA-treated patients (53%) than in the placebo-treated patients (8%)
- Most cases of HFSR in STIVARGA-treated patients appeared during the first cycle of treatment
- The incidences of Grade 3 HFSR (16% vs <1%), Grade 3 rash (3% vs <1%), serious adverse reactions of erythema multiforme (<0.1% vs 0%), and Stevens-Johnson Syndrome (<0.1% vs 0%) were also higher in STIVARGA-treated patients. Across all trials, a higher incidence of HFSR was observed in Asian patients treated with STIVARGA (all grades: 72%; Grade 3: 18%)
- Toxic epidermal necrolysis occurred in 0.02% of 4518 STIVARGA-treated patients across all clinical trials of STIVARGA administered as a single agent
- Withhold STIVARGA, reduce the dose, or permanently discontinue depending on the severity and persistence of dermatologic toxicity
Monitor patients and intervene early
- Conduct a full-body examination of the skin, with emphasis on the palms of the hands and soles of the feet2,3
- HFSR typically occurs early in therapy. Maintain frequent contact with patients to ensure early diagnosis and intervention2
- Other skin conditions, including severe rash, occur at a higher incidence in STIVARGA-treated patients1
- Management of skin toxicities may include temporary treatment interruption and/or dose modification, or, in severe or persistent cases, permanent discontinuation. Institute supportive measures for symptomatic relief1
In the pivotal studies, the following supportive measures were included for HFSR4:
- Control of calluses
- Use of creams (ie, non–urea–based creams, keratolytic creams, alpha hydroxy acids)
- Cushions to protect tender areas
These strategies can be communicated to patients as the “3 C's” for HFSR management.


