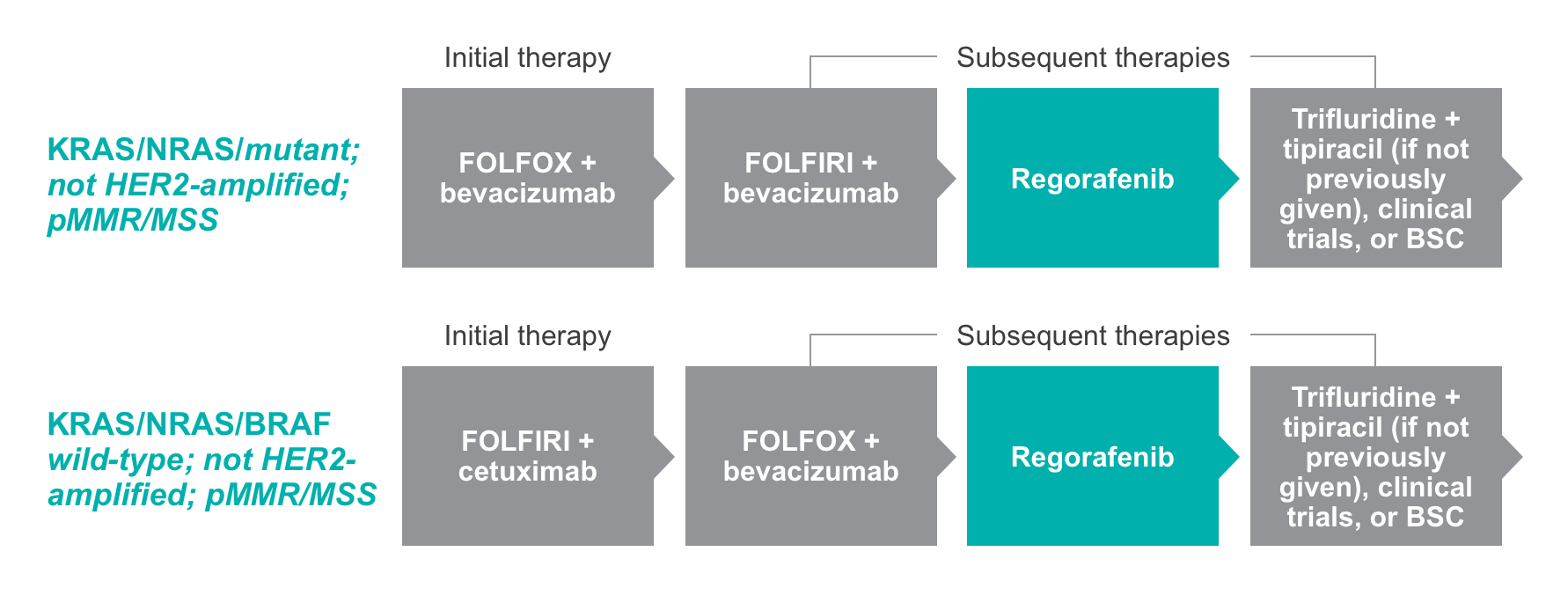
Consider STIVARGA® (regorafenib) in 3rd line after 2 or more standard chemotherapy-containing regimens
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) list regorafenib (STIVARGA) as a potential treatment option for patients who have been previously treated with at least 2 chemo-based therapies (Category 2A)1,2*
Designated Category 2A by the National Comprehensive Cancer Network® (NCCN®).1,2*‡
Adapted with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Colon Cancer V.2.2021 and for Rectal Cancer V.1.2021. © 2021 National Comprehensive Cancer Network, Inc. All rights reserved. The NCCN Guidelines® and illustrations herein may not be reproduced in any form for any purpose without the express written permission of NCCN. To view the most recent and complete version of the NCCN Guidelines, go online to NCCN.org. The NCCN Guidelines are a work in progress that may be refined as often as new significant data become available.
NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
CORRECT (COloRectal cancer treated with REgorafenib or plaCebo after failure of standard Therapy) was a large, international, placebo-controlled, double-blind, randomized trial.4