STIVARGA® (regorafenib) safety profile in CORRECT
AEs reported in ≥10% of mCRC patients treated with STIVARGA and reported more commonly than in patients receiving placebo1*
| STIVARGA (n=500) | ||
|---|---|---|
| AEs | All grades (%) |
Grade ≥3 (%) |
| General disorders and administration site conditions | ||
| Asthenia/fatigue | 64% | 15% |
| Pain | 59% | 9% |
| Fever | 28% | 2% |
| Metabolism and nutrition disorders | ||
| Decreased appetite and food intake | 47% | 5% |
| Skin and subcutaneous tissue disorders | ||
| HFSR/PPES | 45% | 17% |
| Rash† | 26% | 6% |
| Gastrointestinal disorders | ||
| Diarrhea | 43% | 8% |
| Mucositis | 33% | 4% |
| Investigations | ||
| Weight loss | 32% | <1% |
| Infections and infestations | ||
| Infection‡ | 31% | 9% |
| Vascular disorders | ||
| Hypertension | 30% | 8% |
| Hemorrhage‡ | 21% | 2% |
| Respiratory, thoracic, and mediastinal disorders | ||
| Dysphonia | 30% | 0% |
| Nervous system disorders | ||
| Headache | 10% | <1% |
| Placebo (n=253) | ||
|---|---|---|
| AEs | All grades (%) |
Grade ≥3 (%) |
| General disorders and administration site conditions | ||
| Asthenia/fatigue | 46% | 9% |
| Pain | 48% | 7% |
| Fever | 15% | 0% |
| Metabolism and nutrition disorders | ||
| Decreased appetite and food intake | 28% | 4% |
| Skin and subcutaneous tissue disorders | ||
| HFSR/PPES | 7% | 0% |
| Rash† | 4% | <1% |
| Gastrointestinal disorders | ||
| Diarrhea | 17% | 2% |
| Mucositis | 5% | 0% |
| Investigations | ||
| Weight loss | 10% | 0% |
| Infections and infestations | ||
| Infection‡ | 17% | 6% |
| Vascular disorders | ||
| Hypertension | 8% | <1% |
| Hemorrhage‡ | 8% | <1% |
| Respiratory, thoracic, and mediastinal disorders | ||
| Dysphonia | 6% | 0% |
| Nervous system disorders | ||
| Headache | 7% | 0% |
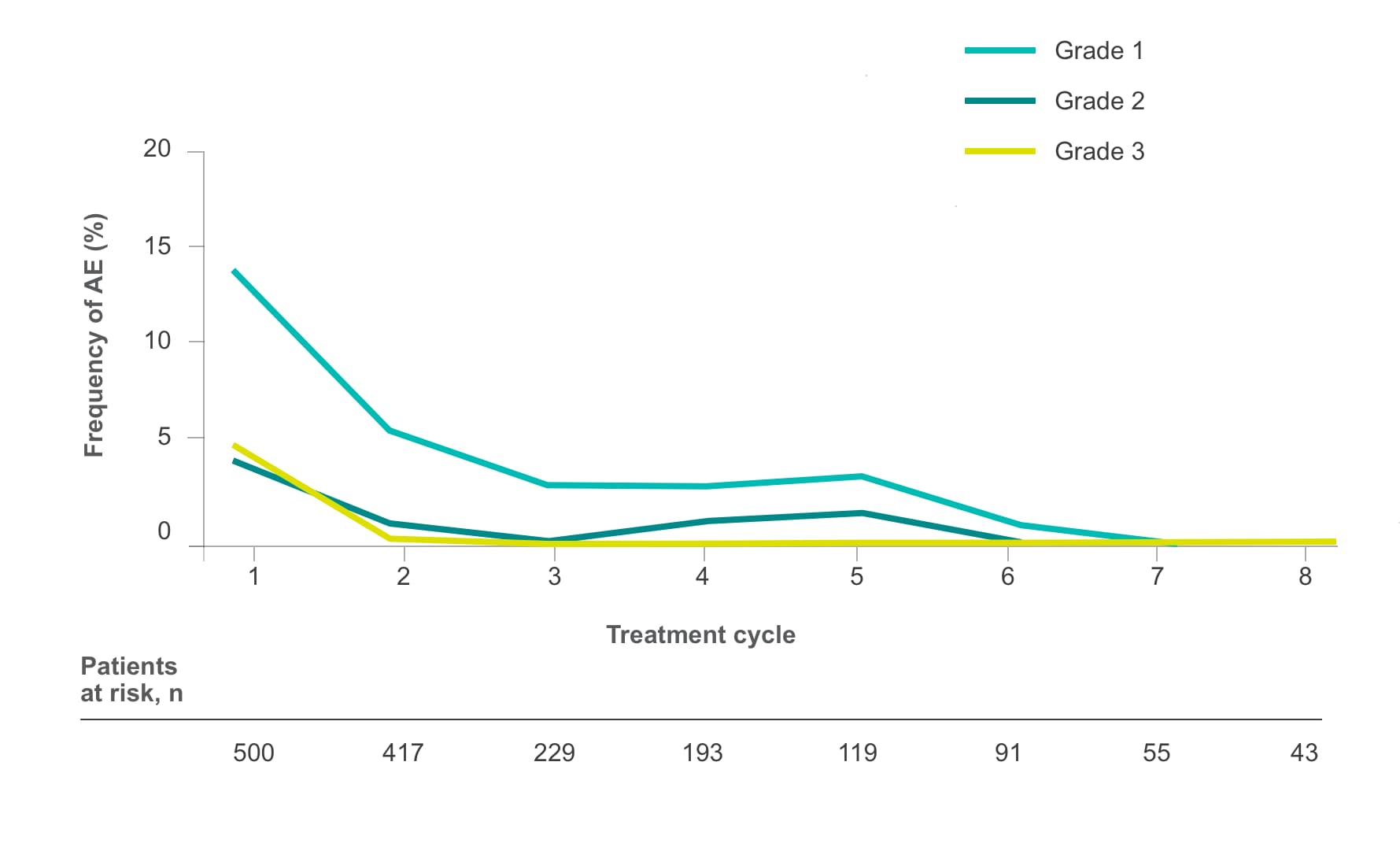
Frequency of select treatment-related AEs ≥Grade 3 occurring in ≥5% of patients in either arm of CORRECT2, 4§
HFSR
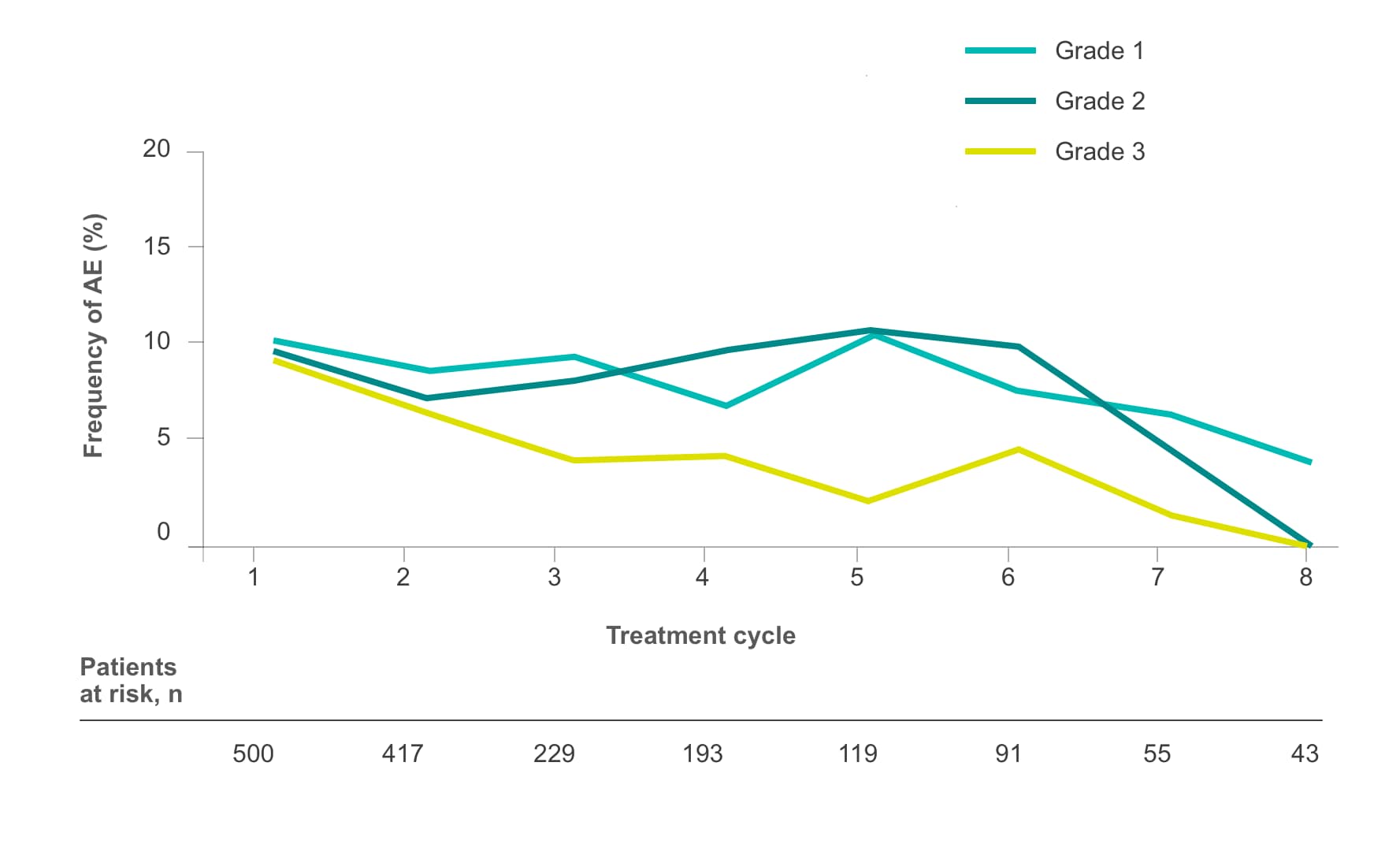
Fatigue
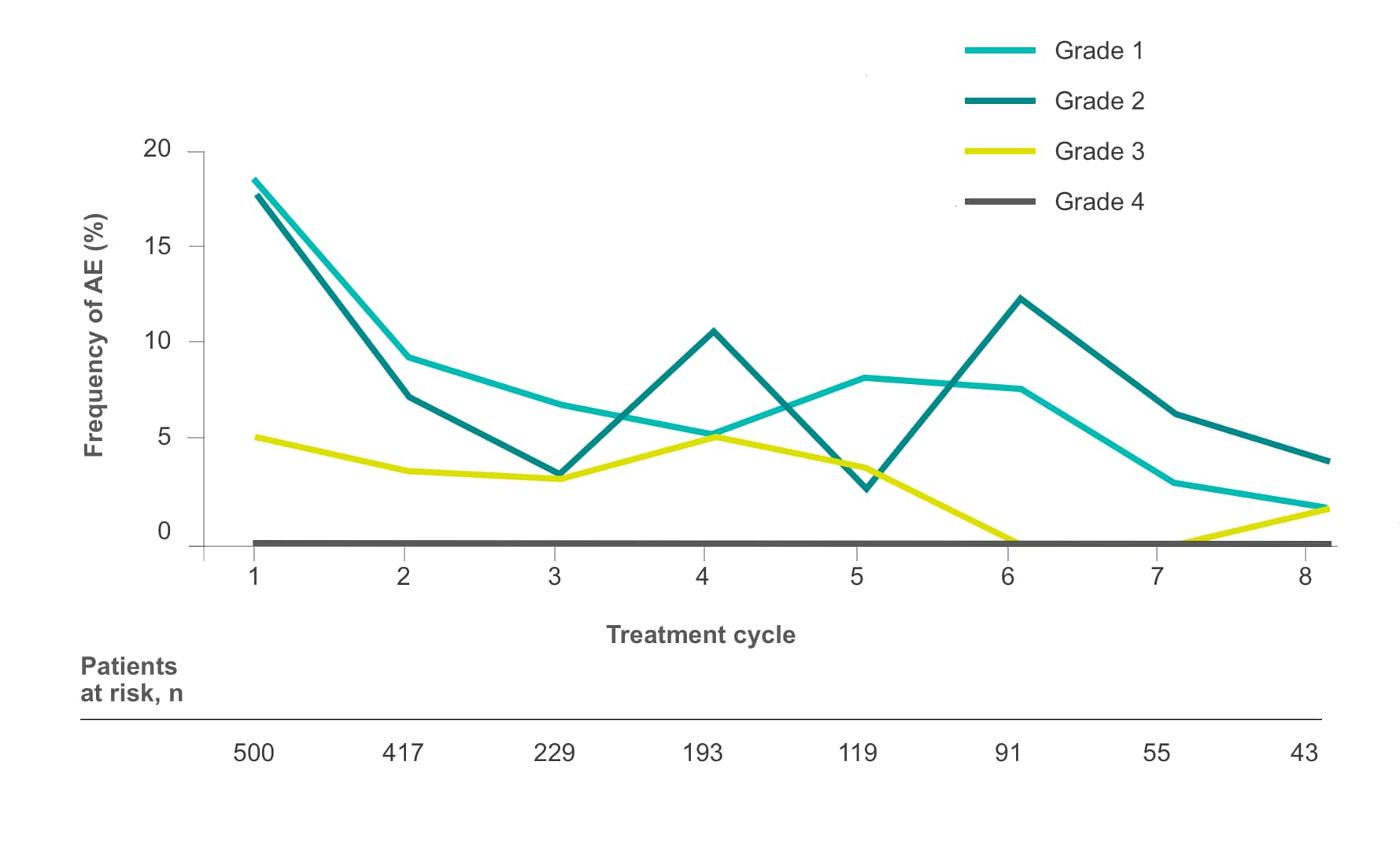
Diarrhea
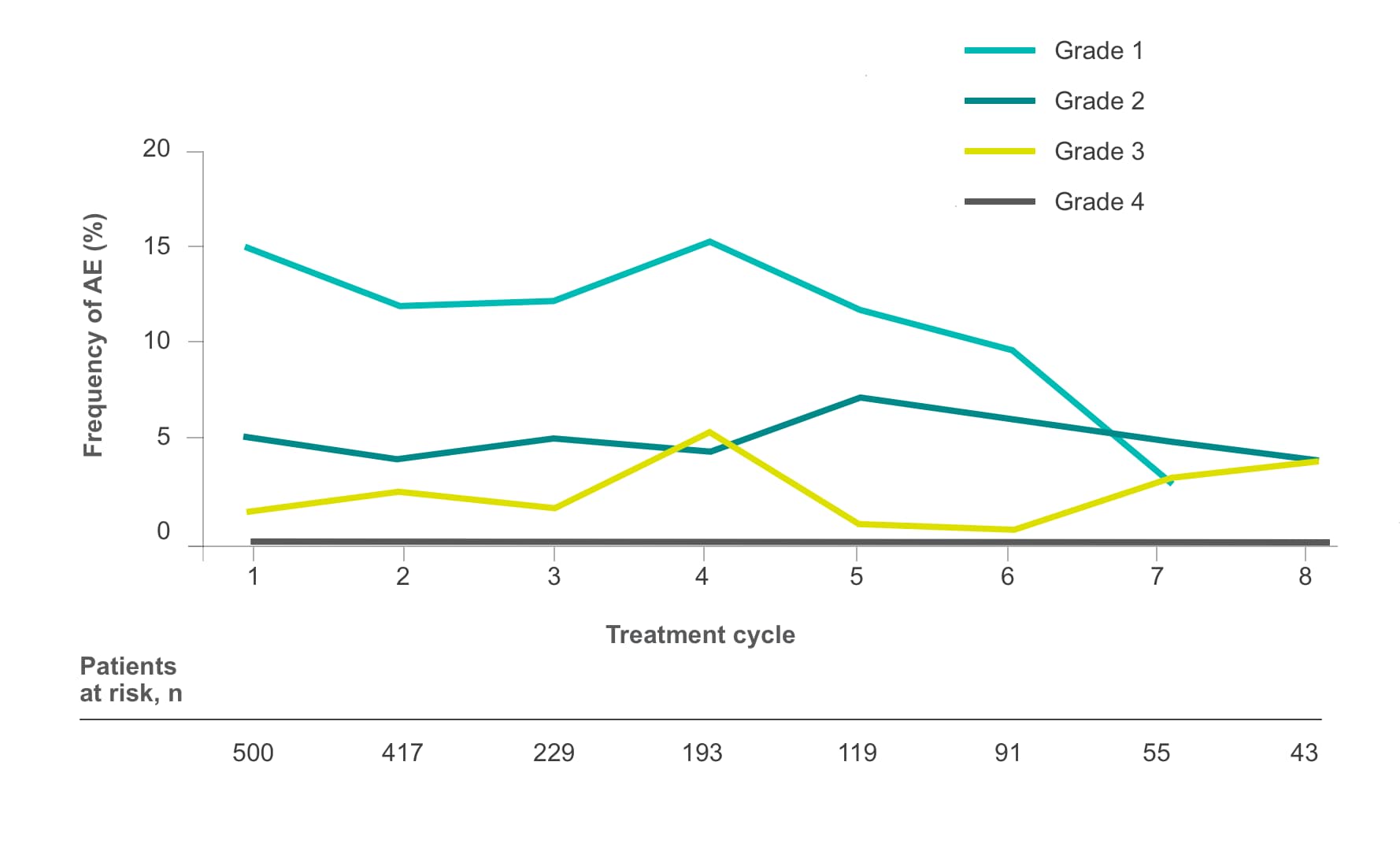
Hypertension
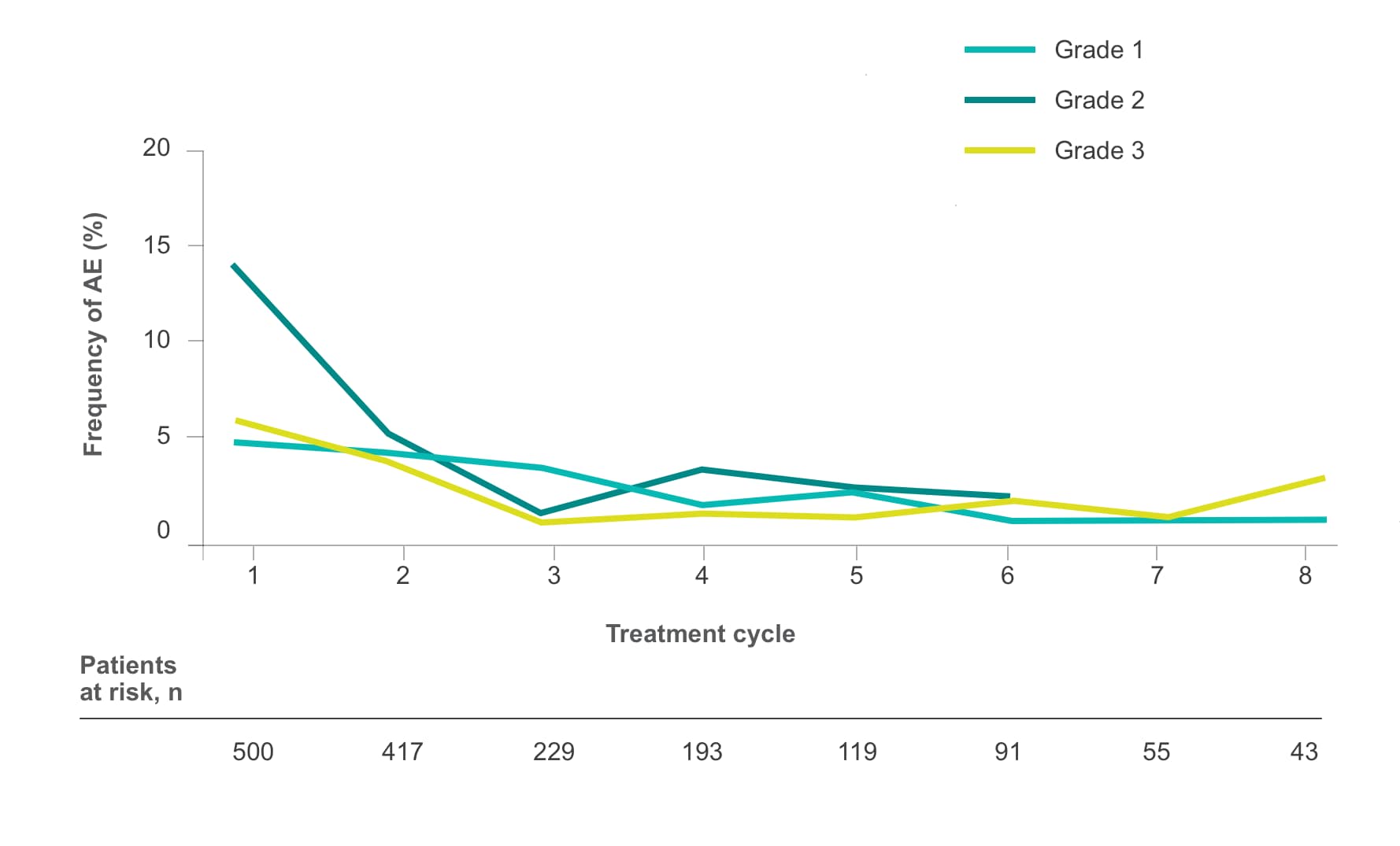
Rash/Desquamation