Harness the proven efficacy of STIVARGA to maximize potential overall survival (OS) for your previously treated patients with mCRC1
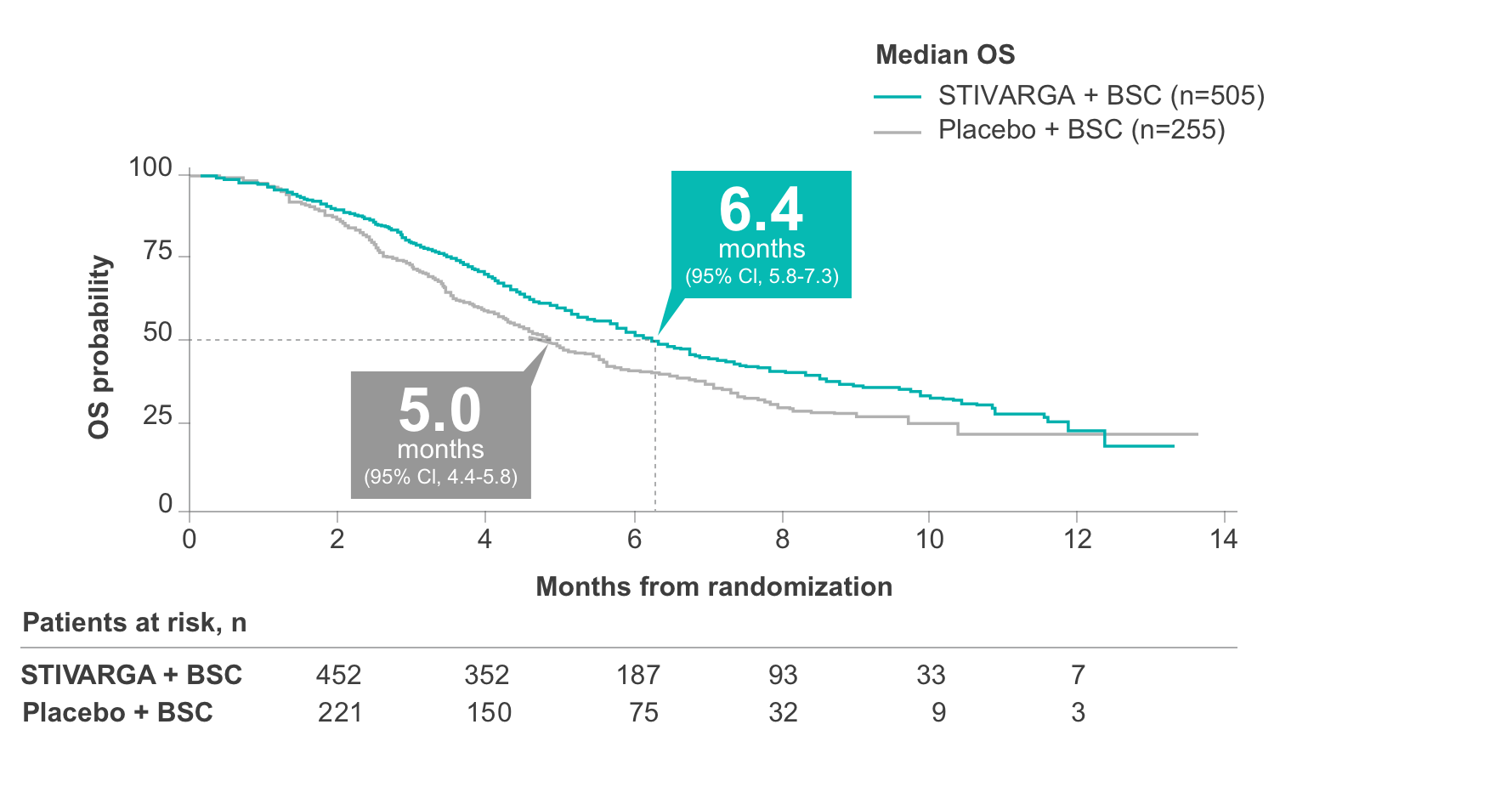
In the pivotal CORRECT trial, STIVARGA demonstrated a median OS of 6.4 months vs 5.0 months with placebo for previously treated patients with mCRC1
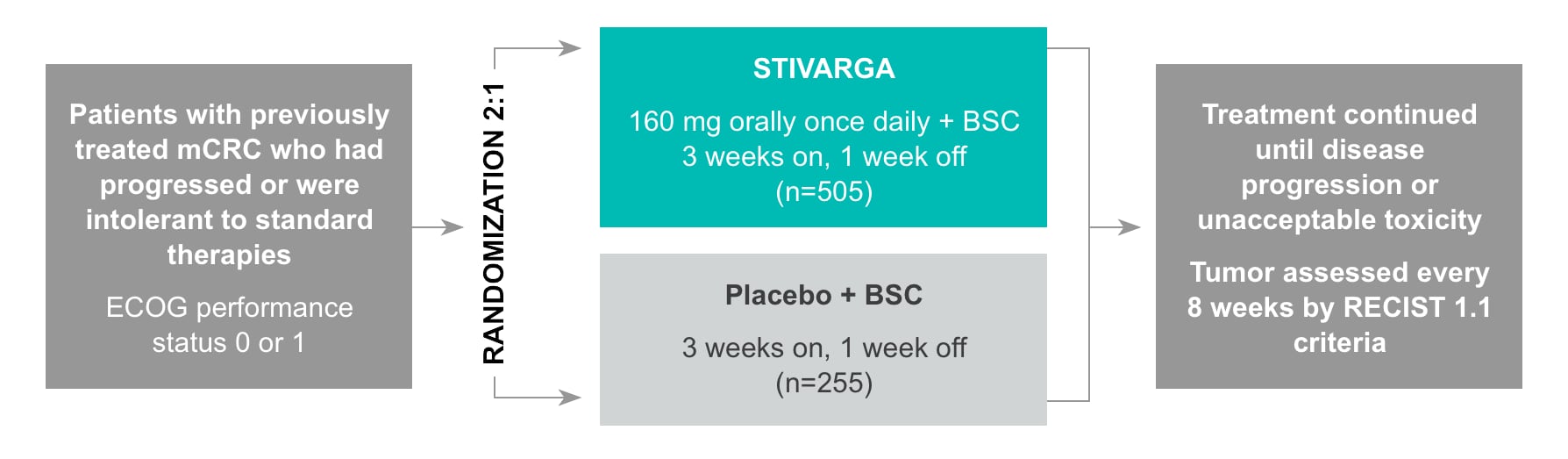
CORRECT (COloRectal cancer treated with REgorafenib or plaCebo after failure of standard Therapy) was a large, international, placebo-controlled, double-blind, randomized (2:1), phase 3 trial that evaluated the efficacy and safety of STIVARGA in patients with mCRC who had progressed after all approved standard therapies (N=760).1, 2
- STIVARGA improved OS in CORRECT, which included patients with historically collected KRAS status (N=729)1
- Historical KRAS status was assessed (59% mutant, 41% KRAS wild-type)
- There were 275 deaths out of 505 patients treated with STIVARGA (55%) vs 157 deaths out of 255 patients treated with placebo (62%)1
Interested in additional results that support the STIVARGA clinical experience?
Cytotoxic therapy in CORRECT
In CORRECT, patients were able to receive cytotoxic therapy following treatment with STIVARGA2,5
CORRECT trial: 26% of patients received cytotoxic therapy after STIVARGA2, 3
| STIVARGA, n (%) | Placebo, n (%) | |
|---|---|---|
| Systemic anticancer treatment during CORRECT trial follow-up | (n=505) | (n=255) |
| Patients with ≥1 medication | 131 (26) | 76 (30) |
| Any antineoplastic or immunomodulation agent | 130 (26) | 74 (29) |
| Systemic anticancer treatment during CORRECT trial follow-up | STIVARGA, n (%) (n=505) | Placebo, n (%) (n=255) |
|---|---|---|
| Patients with ≥1 medication | 131 (26) | 76 (30) |
| Any antineoplastic or immunomodulation agent | 130 (26) | 74 (29) |
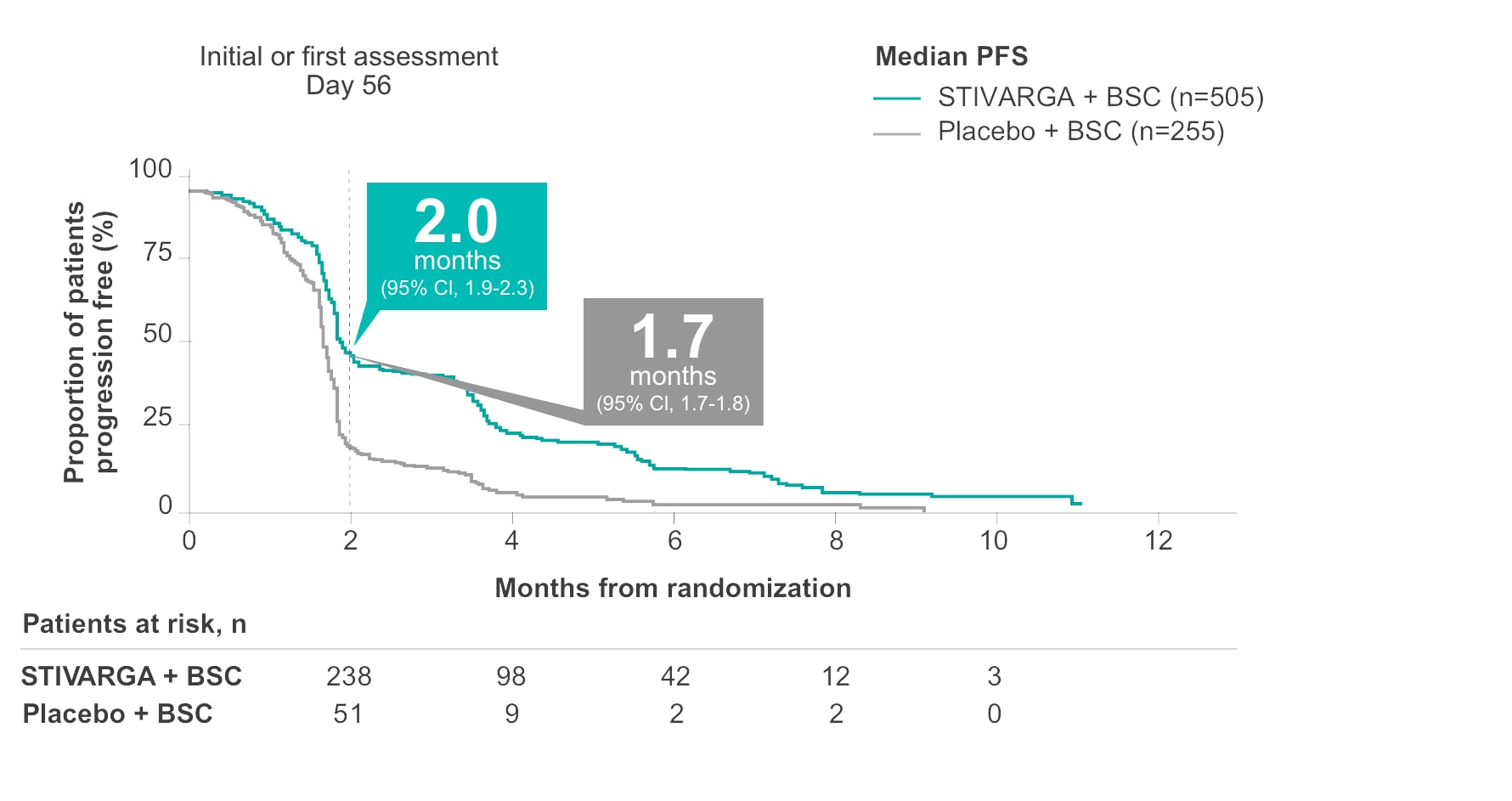
Disease control rate (DCR)
Disease control with 41% of patients treated with STIVARGA vs 15% with placebo2
- DCR included a 41% stable disease rate and a 1% partial response rate in the STIVARGA arm (n=207/505) vs a 15% stable disease rate and a 0.4% partial response rate in the placebo arm (n=38/255)2
- Disease control is defined as proportion of patients with a best response of complete or partial response or stable disease; assessment of stable disease had to be made at least 6 weeks after randomization
CORRECT trial study design
Multicenter, randomized, double-blind, placebo-controlled, phase 3 trial2
- Study arms abbreviated as STIVARGA and placebo throughout this website
- Stratification: Prior treatment with anti-VEGF drugs, time from diagnosis of mCRC, and geographical region2
- Treatment continued until disease progression or unacceptable toxicity2
- Tumors were assessed every 8 weeks by RECIST 1.1 criteria2
- No crossover between treatment groups was allowed2
CORRECT trial patient population
Most demographics and disease characteristics were similar between arms in the CORRECT trial2
Baseline characteristics2
| STIVARGA (n=505) | Placebo (n=255) | |
|---|---|---|
| Median age, y | 61 | 61 |
| Sex | ||
| Male | 62% | 60% |
| Female | 38% | 40% |
| Disease site | ||
| Colon | 64% | 68% |
| Rectum | 30% | 27% |
| Both | 6% | 5% |
| Historical KRAS status | ||
| Mutated | 54% | 62% |
| Race | ||
| White | 78% | 79% |
| Black or African American | 1% | 3% |
| Asian | 15% | 14% |
| Other or not specified | 6% | 4% |
| ECOG performance status | ||
| ECOG PS 0 | 52% | 57% |
| ECOG PS 1 | 48% | 43% |
STAR-T Data: Complementary Results
Exploring the use of regorafenib in real-world¶ patients who no longer benefit from IV chemotherapy1,6
¶RWE complements data from randomized clinical trials. It is important to understand both the randomized clinical trial results and the limitations of retrospective real world studies. Observational retrospective analyses are not intended for direct comparison with clinical trials.
STUDY DESIGN
Data source6
- STAR-T was a retrospective cohort study (N=818)
- Data source was the Flatiron Health electronic heath record database
- The Flatiron dataset was derived from mCRC patient charts from more than 280 cancer clinics across the US
Inclusion criteria6
- Patients were aged ≥18 years at the time of mCRC diagnosis
- Patients received sequential treatment with regorafenib followed by trifluridine/tipiracil ± bevacizumab (R-T) or vice versa (T-R), from January 2015 to November 2022
- Patients were divided into 2 cohorts: R-T (n=393) or T-R (n=425) and stratified by line of treatment (third or fourth line)
Objectives6
- Primary objective: to describe demographic, clinical characteristics, and biomarkers of patients who received sequential treatment with R-T or T-R
- Secondary objectives: to describe subsequent therapies, frequency and incidence of neutropenia and myelosuppression-related medical interventions, duration of sequential treatment, and OS of patients treated with R-T or T-R
STUDY LIMITATIONS6
- This was a retrospective analysis based on health records, which may have missing or erroneous data entry
- Given the nature of the information, progression was not centrally assessed
- Dosing information was not collected as part of the study
Secondary objectives of STAR-T study in patients with mCRC treated sequentially with regorafenib
Secondary objectives: OS and subsequent therapies6
STAR-T FINDINGS2,6
Safety Findings6
39% of patients who started sequential therapy with regorafenib reported neutropenia
14% of patients who started sequential therapy with regorafenib used G-CSF