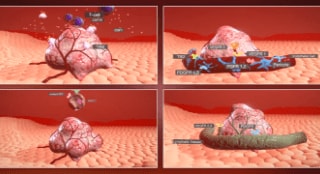
10 - Can you describe the mechanism of action for STIVARGA?
Can you describe the mechanism of action for STIVARGA?
2024-12-10
Medical Director, Division of Ambulatory Treatment Centers, MD Anderson Cancer Center (MDACC),
The University of Texas, MDACC,
Houston, TX