The information provided in this section is intended expressly for healthcare professionals in the United States. Click “OK” to enter if you are a US healthcare professional.
Meet Jane
Return to all patient cases
*Not based on actual patient.
Meet Jane:
Age 60
Stage IV recurrent colon cancer
KRAS wild-type
Meet Jane*
Jane is a 60-year-old woman who was diagnosed with stage IIb, KRAS wild-type, right-sided colon cancer. The cancer returned 3 years after completing adjuvant capecitabine with unresectable lung and liver metastases.
Disease history
- Following diagnosis with stage IIb, right-sided colon cancer, she was successfully treated with total resection and 6 months of adjuvant capecitabine
- Three years following the completion of adjuvant therapy, she was found to have unresectable lung and liver metastases
- KRAS wild-type with no evidence of microsatellite instability or defective mismatch repair
- She was treated with FOLFOX + bevacizumab regimen and had PFS of 8 months
- She was subsequently treated with irinotecan + cetuximab for 5 months until disease progression
Relevant medical history
- Hypertension well controlled with diuretic/beta-blocker therapy (BP: 116/78 mm Hg)
- ECOG performance status: 1
Treatment options for this patient
EXPANDED VIEW
A possible treatment pathway for Jane1†
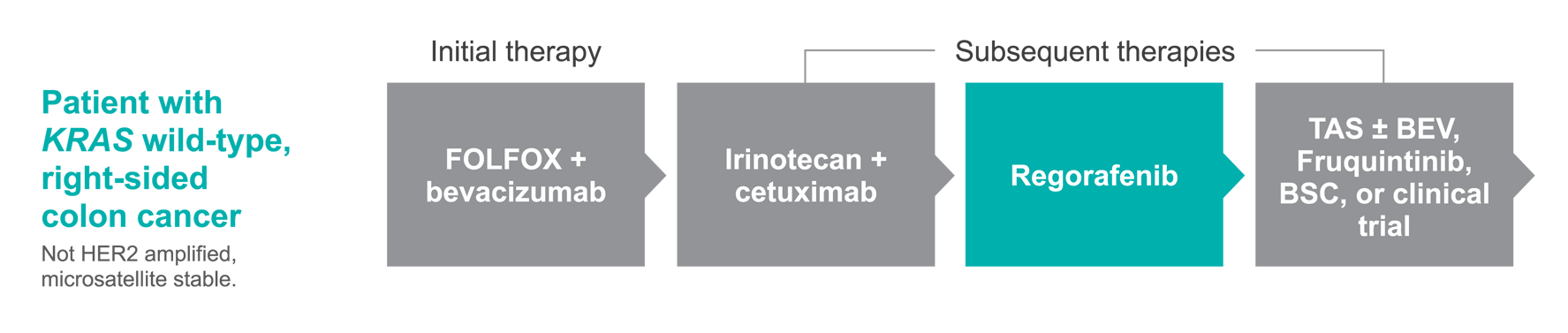
The treatment regimens shown represent just 1 possible treatment approach for this patient. According to NCCN Guidelines®, a number of alternative treatment plans could also be used.
NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
Adapted with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Colon Cancer V.2.2024. © 2024 National Comprehensive Cancer Network, Inc. All rights reserved. The NCCN Guidelines® and illustrations herein may not be reproduced in any form for any purpose without the express written permission of NCCN. To view the most recent and complete version of the NCCN Guidelines, go online to NCCN.org. The NCCN Guidelines are a work in progress that may be refined as often as new significant data becomes available.
ACT IN TIME in previously treated patients with mCRC to help the survival potential of their treatment journey2,3
STIVARGA® (regorafenib) has been shown to be effective in patients with mCRC who have been previously treated with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy, an anti-VEGF therapy, and if KRAS wild-type, an anti-EGFR therapy.2,3
Significant improvement in overall survival (OS) in a phase 3 trial
- In the pivotal CORRECT trial, STIVARGA demonstrated a 6.4-month (95% CI, 5.8-7.3) overall survival (OS) rate in previously treated patients with mCRC, compared to 5.0 months (95% CI, 4.4-5.8) for placebo2
- 23% reduction in the risk of death with STIVARGA (HR=0.77 [95% CI, 0.64-0.94; P=0.0102])2
- There were 275 deaths out of 505 patients treated with STIVARGA (55%) vs 157 deaths out of 255 patients treated with placebo (62%)2
Significant improvement in progression-free survival (PFS) in a phase 3 trial
- In the CORRECT trial, STIVARGA was associated with a 51% reduction in risk of progression or death compared to placebo (HR=0.49 [95% CI, 0.42-0.58; P<0.0001])2
- Median PFS was 2.0 months (95% CI, 1.9-2.3) compared to 1.7 months (95% CI, 1.7-1.8) for placebo2
- There were 417 deaths out of 505 patients treated with STIVARGA (83%) vs 231 deaths out of 255 patients treated with placebo (91%)2
Considerations when starting patients on STIVARGA
- Select inclusion criteria3:
- Eastern Cooperative Oncology Group (ECOG) performance status ≤1
- Life expectancy of at least 3 months
- Adequate bone marrow, liver, and renal function
- Help patients reach the first tumor assessment by monitoring adverse events frequently3,4
- Monitor adverse events within the first week and every 2 weeks thereafter, or more often if needed
In the CORRECT trial3:
- 27% (n=135) and 25% (n=63) of patients received ≤2 lines of prior systemic therapy in the STIVARGA (n=505) and placebo (n=255) arms, respectively
- 26% of patients received cytotoxic therapy after STIVARGA
CORRECT (COloRectal cancer treated with REgorafenib or plaCebo after failure of standard Therapy) was a large, international, placebo-controlled, double-blind, randomized (2:1), phase 3 trial that evaluated the efficacy and safety of STIVARGA in patients with mCRC who had progressed after all approved standard therapies (N=760).2,3
Indications
STIVARGA is indicated for the treatment of patients with metastatic colorectal cancer (CRC) who have been previously treated with fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapy, an anti-VEGF therapy, and, if RAS wild-type, an anti-EGFR therapy.
STIVARGA is indicated for the treatment of patients with locally advanced, unresectable or metastatic gastrointestinal stromal tumor (GIST) who have been previously treated with imatinib mesylate and sunitinib malate.
STIVARGA is indicated for the treatment of patients with hepatocellular carcinoma (HCC) who have been previously treated with sorafenib.
Important Safety Information
WARNING: HEPATOTOXICITY
- Severe and sometimes fatal hepatotoxicity has occurred in clinical trials.
- Monitor hepatic function prior to and during treatment.
- Interrupt and then reduce or discontinue STIVARGA for hepatotoxicity as manifested by elevated liver function tests or hepatocellular necrosis, depending upon severity and persistence.
Hepatotoxicity: Severe drug-induced liver injury with fatal outcome occurred in STIVARGA-treated patients across all clinical trials. In most cases, liver dysfunction occurred within the first 2 months of therapy and was characterized by a hepatocellular pattern of injury. In metastatic colorectal cancer (mCRC), fatal hepatic failure occurred in 1.6% of patients in the STIVARGA arm and in 0.4% of patients in the placebo arm. In gastrointestinal stromal tumor (GIST), fatal hepatic failure occurred in 0.8% of patients in the STIVARGA arm. In hepatocellular carcinoma (HCC), there was no increase in the incidence of fatal hepatic failure as compared to placebo.
Liver Function Monitoring: Obtain liver function tests (ALT, AST, and bilirubin) before initiation of STIVARGA and monitor at least every 2 weeks during the first 2 months of treatment. Thereafter, monitor monthly or more frequently as clinically indicated. Monitor liver function tests weekly in patients experiencing elevated liver function tests until improvement to less than 3 times the upper limit of normal (ULN) or baseline values. Temporarily hold and then reduce or permanently discontinue STIVARGA, depending on the severity and persistence of hepatotoxicity as manifested by elevated liver function tests or hepatocellular necrosis.
Infections: STIVARGA caused an increased risk of infections. The overall incidence of infection (Grades 1-5) was higher (32% vs 17%) in 1142 STIVARGA-treated patients as compared to the control arm in randomized placebo-controlled trials. The incidence of grade 3 or greater infections in STIVARGA treated patients was 9%. The most common infections were urinary tract infections (5.7%), nasopharyngitis (4.0%), mucocutaneous and systemic fungal infections (3.3%) and pneumonia (2.6%). Fatal outcomes caused by infection occurred more often in patients treated with STIVARGA (1.0%) as compared to patients receiving placebo (0.3%); the most common fatal infections were respiratory (0.6% vs 0.2%). Withhold STIVARGA for Grade 3 or 4 infections, or worsening infection of any grade. Resume STIVARGA at the same dose following resolution of infection.
Hemorrhage: STIVARGA caused an increased incidence of hemorrhage. The overall incidence (Grades 1-5) was 18.2% in 1142 patients treated with STIVARGA vs 9.5% with placebo in randomized, placebo-controlled trials. The incidence of grade 3 or greater hemorrhage in patients treated with STIVARGA was 3.0%. The incidence of fatal hemorrhagic events was 0.7%, involving the central nervous system or the respiratory, gastrointestinal, or genitourinary tracts. Permanently discontinue STIVARGA in patients with severe or life-threatening hemorrhage and monitor INR levels more frequently in patients receiving warfarin.
Gastrointestinal Perforation or Fistula: Gastrointestinal perforation occurred in 0.6% of 4518 patients treated with STIVARGA across all clinical trials of STIVARGA administered as a single agent; this included eight fatal events. Gastrointestinal fistula occurred in 0.8% of patients treated with STIVARGA and in 0.2% of patients in the placebo arm across randomized, placebo-controlled trials. Permanently discontinue STIVARGA in patients who develop gastrointestinal perforation or fistula.
Dermatological Toxicity: In randomized, placebo-controlled trials, adverse skin reactions occurred in 71.9% of patients with STIVARGA arm and 25.5% of patients in the placebo arm including hand-foot skin reaction (HFSR) also known as palmar-plantar erythrodysesthesia syndrome (PPES) and severe rash, requiring dose modification. In the randomized, placebo-controlled trials, the overall incidence of HFSR was higher in 1142 STIVARGA-treated patients (53% vs 8%) than in the placebo-treated patients. Most cases of HFSR in STIVARGA-treated patients appeared during the first cycle of treatment. The incidences of Grade 3 HFSR (16% vs <1%), Grade 3 rash (3% vs <1%), serious adverse reactions of erythema multiforme (<0.1% vs 0%), and Stevens-Johnson syndrome (<0.1% vs 0%) were higher in STIVARGA-treated patients. Across all trials, a higher incidence of HFSR was observed in Asian patients treated with STIVARGA (all grades: 72%; Grade 3: 18%). Toxic epidermal necrolysis occurred in 0.02% of 4518 STIVARGA-treated patients across all clinical trials of STIVARGA administered as a single agent. Withhold STIVARGA, reduce the dose, or permanently discontinue depending on the severity and persistence of dermatologic toxicity.
Hypertension: Hypertensive crisis occurred in 0.2% in STIVARGA-treated patients and in none of the patients in placebo arm across all randomized, placebo-controlled trials. STIVARGA caused an increased incidence of hypertension (30% vs 8% in mCRC, 59% vs 27% in GIST, and 31% vs 6% in HCC). The onset of hypertension occurred during the first cycle of treatment in most patients who developed hypertension (67% in randomized, placebo controlled trials). Do not initiate STIVARGA until blood pressure is adequately controlled. Monitor blood pressure weekly for the first 6 weeks of treatment and then every cycle, or more frequently, as clinically indicated. Temporarily or permanently withhold STIVARGA for severe or uncontrolled hypertension.
Cardiac Ischemia and Infarction: STIVARGA increased the incidence of myocardial ischemia and infarction (0.9% with STIVARGA vs 0.2% with placebo) in randomized placebo-controlled trials. Withhold STIVARGA in patients who develop new or acute cardiac ischemia or infarction, and resume only after resolution of acute cardiac ischemic events if the potential benefits outweigh the risks of further cardiac ischemia.
Reversible Posterior Leukoencephalopathy Syndrome (RPLS): Reversible posterior leukoencephalopathy syndrome (RPLS), a syndrome of subcortical vasogenic edema diagnosed by characteristics finding on MRI, occurred in one of 4800 STIVARGA-treated patients across all clinical trials. Perform an evaluation for RPLS in any patient presenting with seizures, severe headache, visual disturbances, confusion, or altered mental function. Discontinue STIVARGA in patients who develop RPLS.
Wound Healing Complications: Impaired wound healing complications can occur in patients who receive drugs that inhibit the VEGF signaling pathway. Therefore, STIVARGA has the potential to adversely affect wound healing. Withhold STIVARGA for at least 2 weeks prior to elective surgery. Do not administer for at least 2 weeks following major surgery and until adequate wound healing. The safety of resumption of STIVARGA after resolution of wound healing complications has not been established.
Embryo-Fetal Toxicity: STIVARGA can cause fetal harm when administered to a pregnant woman. There are no available data on STIVARGA use in pregnant women. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential and males with female partners of reproductive potential to use effective contraception during treatment with STIVARGA and for 2 months after the final dose.
Nursing Mothers: Because of the potential for serious adverse reactions in breastfed infants from STIVARGA, do not breastfeed during treatment with STIVARGA and for 2 weeks after the final dose.
Most Frequently Observed Adverse Drug Reactions in mCRC (≥30%): The most frequently observed adverse drug reactions (≥30%) in STIVARGA-treated patients vs placebo-treated patients in mCRC, respectively, were: asthenia/fatigue (64% vs 46%), pain (59% vs 48%), decreased appetite and food intake (47% vs 28%), HFSR/PPE (45% vs 7%), diarrhea (43% vs 17%), mucositis (33% vs 5%), weight loss (32% vs 10%), infection (31% vs 17%), hypertension (30% vs 8%), and dysphonia (30% vs 6%).
Most Frequently Observed Adverse Drug Reactions in GIST (≥30%): The most frequently observed adverse drug reactions (≥30%) in STIVARGA-treated patients vs placebo-treated patients in GIST, respectively, were: HFSR/PPE (67% vs 12%), pain (60% vs 55%), hypertension (59% vs 27%), asthenia/fatigue (52% vs 39%), diarrhea (47% vs 9%), mucositis (40% vs 8%), dysphonia (39% vs 9%), infection (32% vs 5%), decreased appetite and food intake (31% vs 21%), and rash (30% vs 3%).
Most Frequently Observed Adverse Drug Reactions in HCC (≥30%): The most frequently observed adverse drug reactions (≥30%) in STIVARGA-treated patients vs placebo-treated patients in HCC, respectively, were: pain (55% vs 44%), HFSR/PPE (51% vs 7%), asthenia/fatigue (42% vs 33%), diarrhea (41% vs 15%), hypertension (31% vs 6%), infection (31% vs 18%), decreased appetite and food intake (31% vs 15%).
For important risk and use information, please see the full Prescribing Information including the Boxed Warning.
You are encouraged to report side effects or quality complaints of products to the FDA by visiting www.fda.gov/medwatch or calling 1-800-FDA-1088. For Bayer products, you can report these directly to Bayer by clicking here.
†For a complete listing of treatment options, see NCCN.org.
BP, blood pressure; BSC, best supportive care; CT, computed tomography; EGFR, epidermal growth factor receptor; FOLFIRI, folinic acid, fluorouracil, and irinotecan; FOLFOX, folinic acid, fluorouracil, and oxaliplatin; mCRC, metastatic colorectal cancer; NCCN, National Comprehensive Cancer Network; VEGF, vascular endothelial growth factor.
References:
- Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Colon Cancer. V.2.2024. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed May 24, 2024. To view the most recent and complete version of the guideline, go online to NCCN.org. Return to content
- STIVARGA [prescribing information]. Whippany, NJ: Bayer HealthCare Pharmaceuticals, Inc; February 2020. Return to content
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303-312. Return to content
- Grothey A, George S, Van Cutsem E, Blay JY, Sobrero A, Demetri GD. Optimizing treatment outcomes with regorafenib: personalized dosing and other strategies to support patient care. Oncologist. 2014;19(6):669-680. Return to content


